Fusion Detection

Genomic Instability
Liquid Biopsy

Long-read Sequencing
Targeted Panels
Welcome tos-Test Genomics
standardising genomic testing
Inline with the National Genomic Strategy
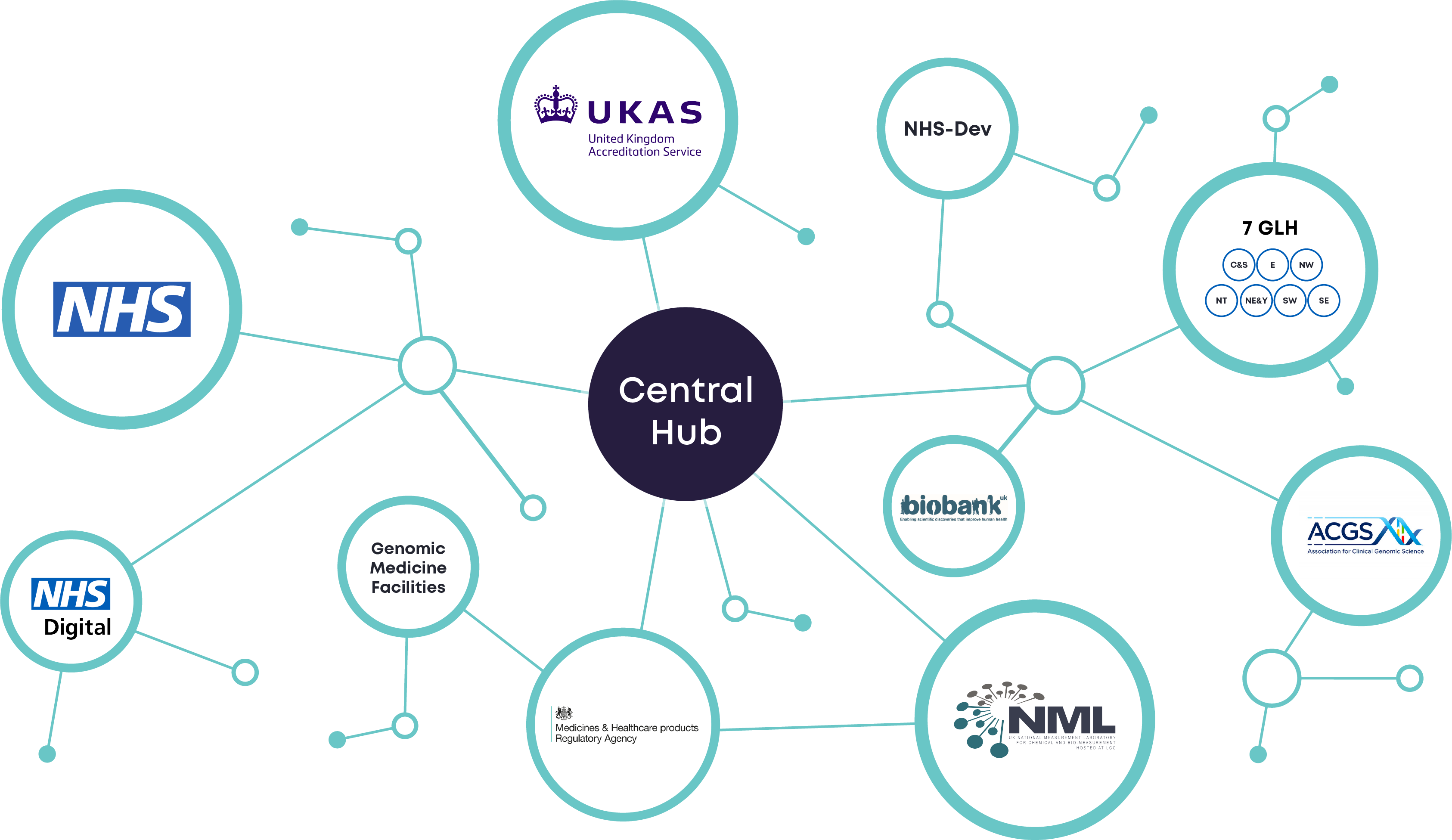
We're Connected!
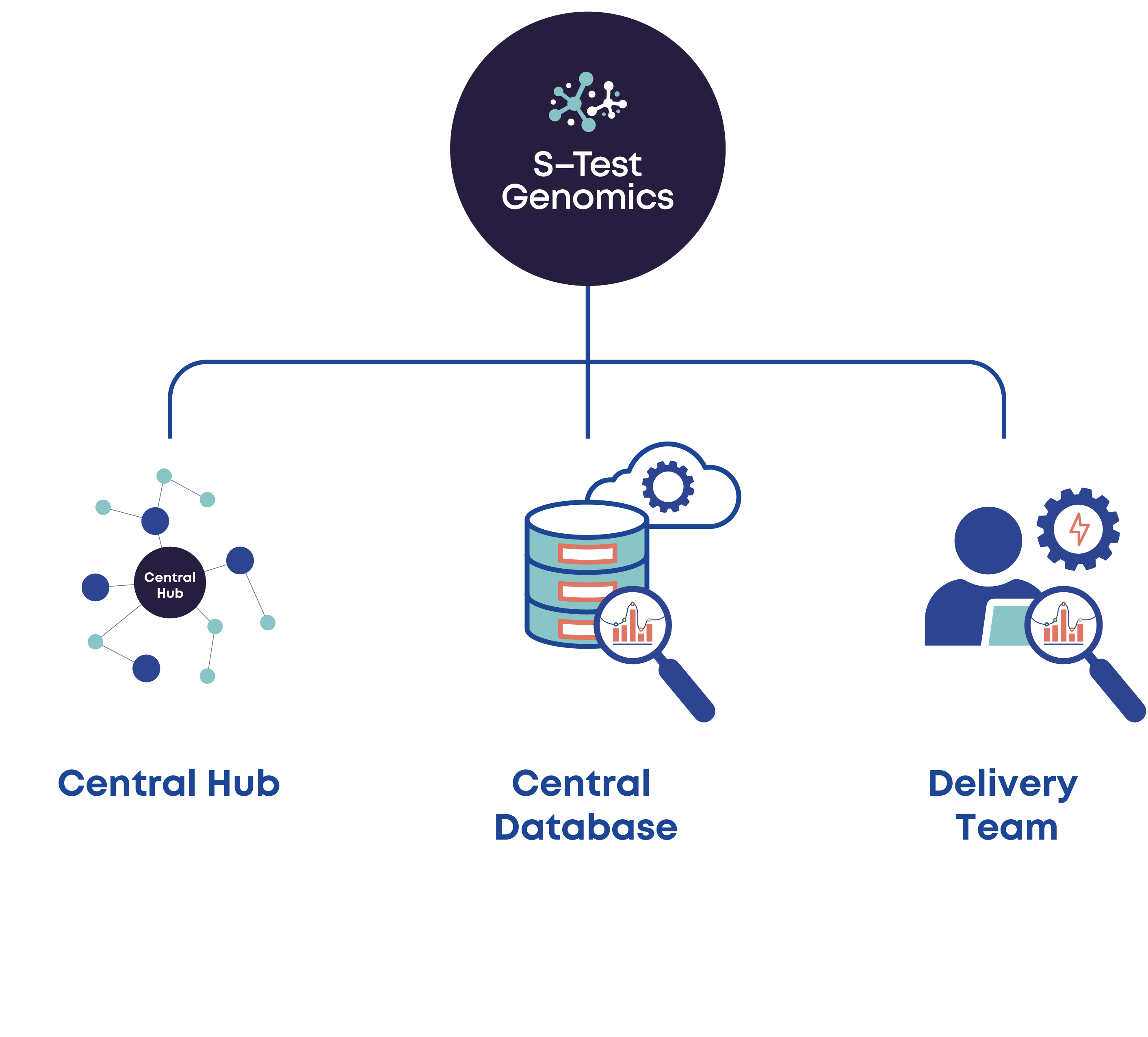
s-Test Genomics components
- A central hub of multidisciplinary expert advisors
- Cloud informatics infrastructure for data exchange, storage, and analysis
- data scientists, design architects, statisticians
- Policy and ethical advisors
- Project administrators
A central hub of multidisciplinary experts working collaboratively to fulfill the safe and timely implementation of the NHS genomic strategy.
S-Test Genomics is a collaborative initiative between key organizations, including the NHS, UKAS, MHRA and the National Measurement Laboratory (NML) which was developed through round 3 of the NHS CSO KTP program 2021 to build long-term partnerships between clinical, research and industry teams through collaboration at a senior level. Start-up funding was provided by the Cancer Research Horizons (CRH) innovative entrepreneur program in collaboration with Innovate UK.
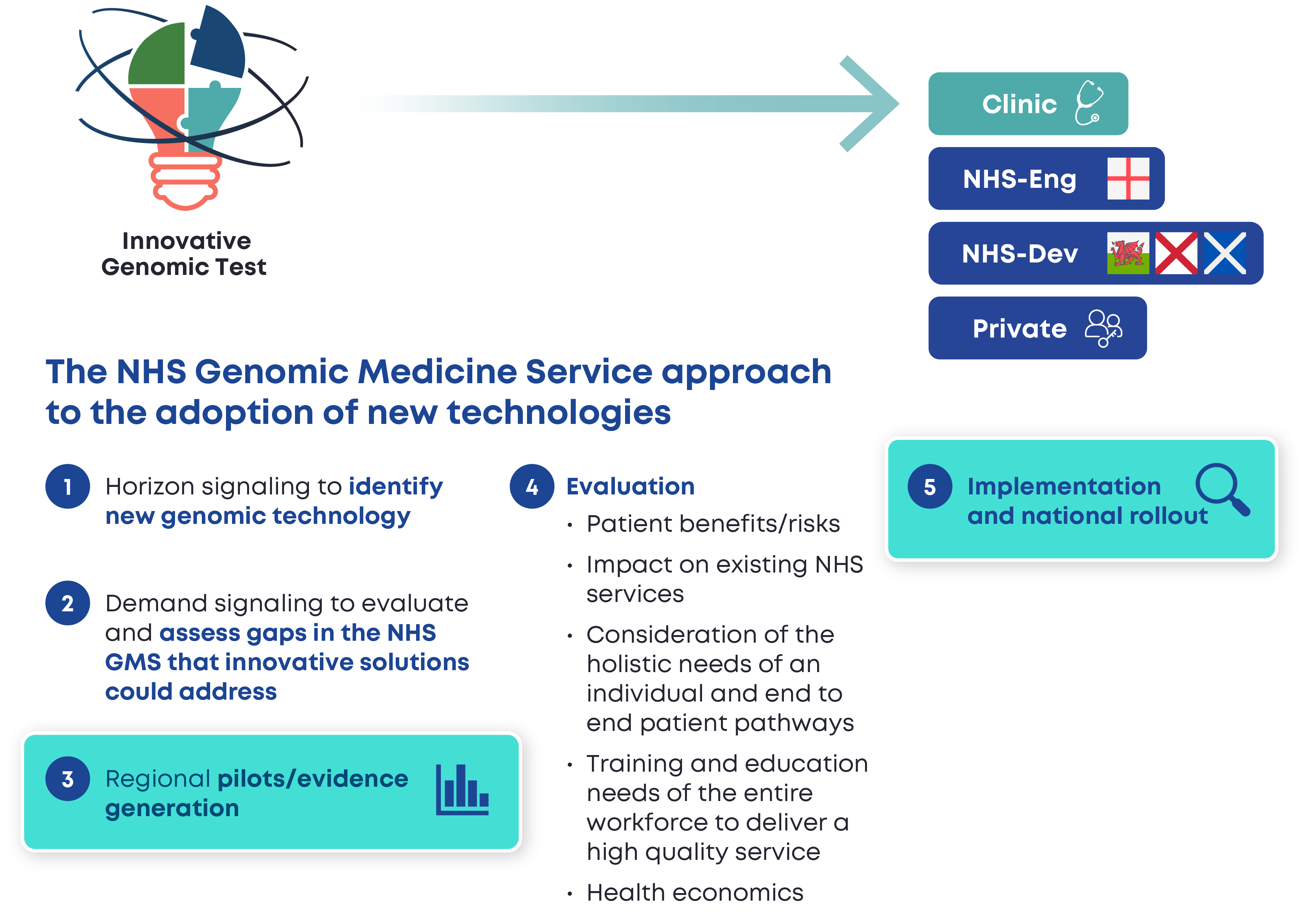
Activities
Interlaboratory comparisons and contingency
Method validation review and design
Education & Good Practice Guidelines
Technology benchmarking
Evidence-based communication
About us
Vision & Mission
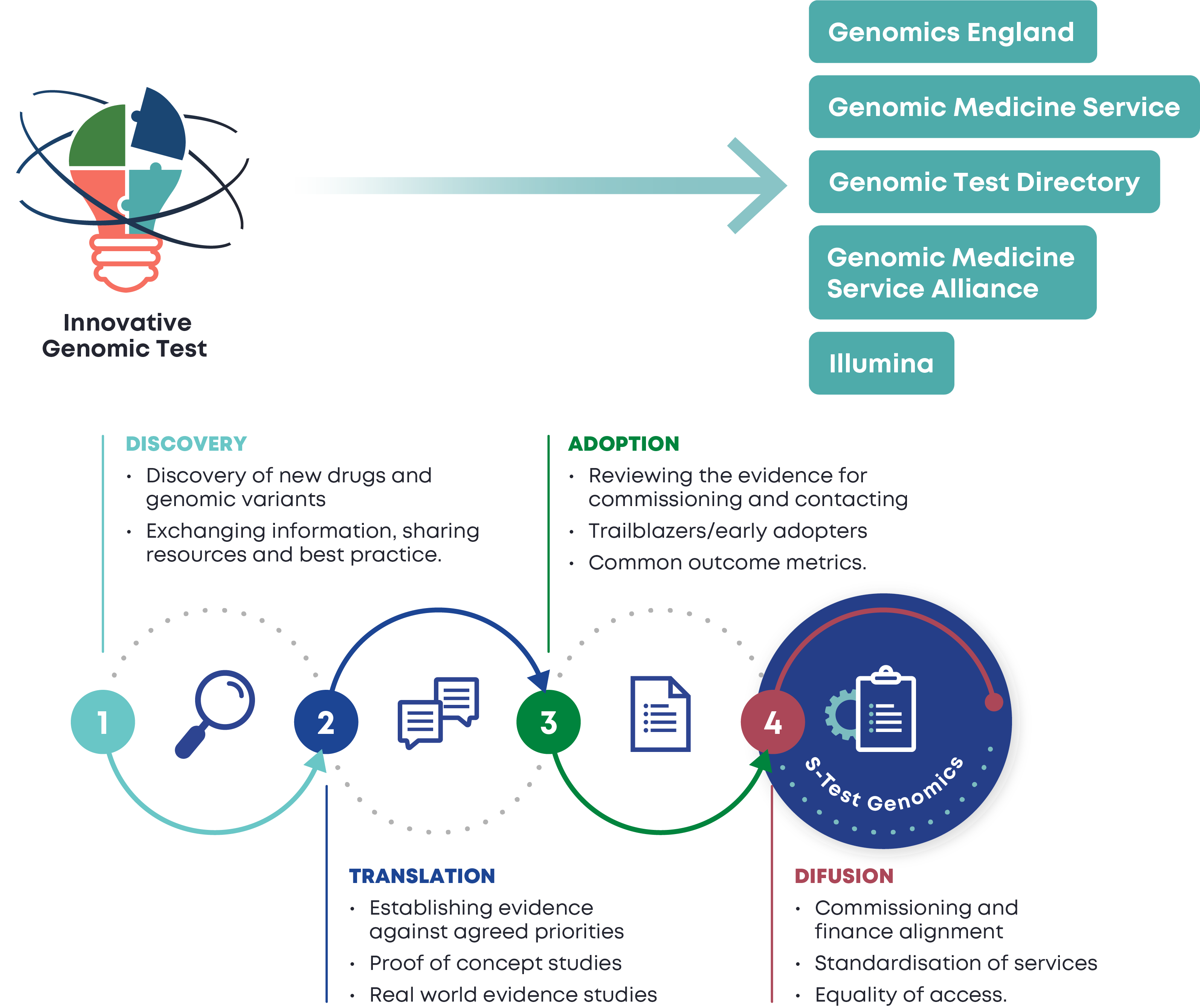
s-Test Genomics vision is to expedite patient access to cutting-edge genomic innovations, at the same time, ensuring the process is safe and fit-for-purpose.
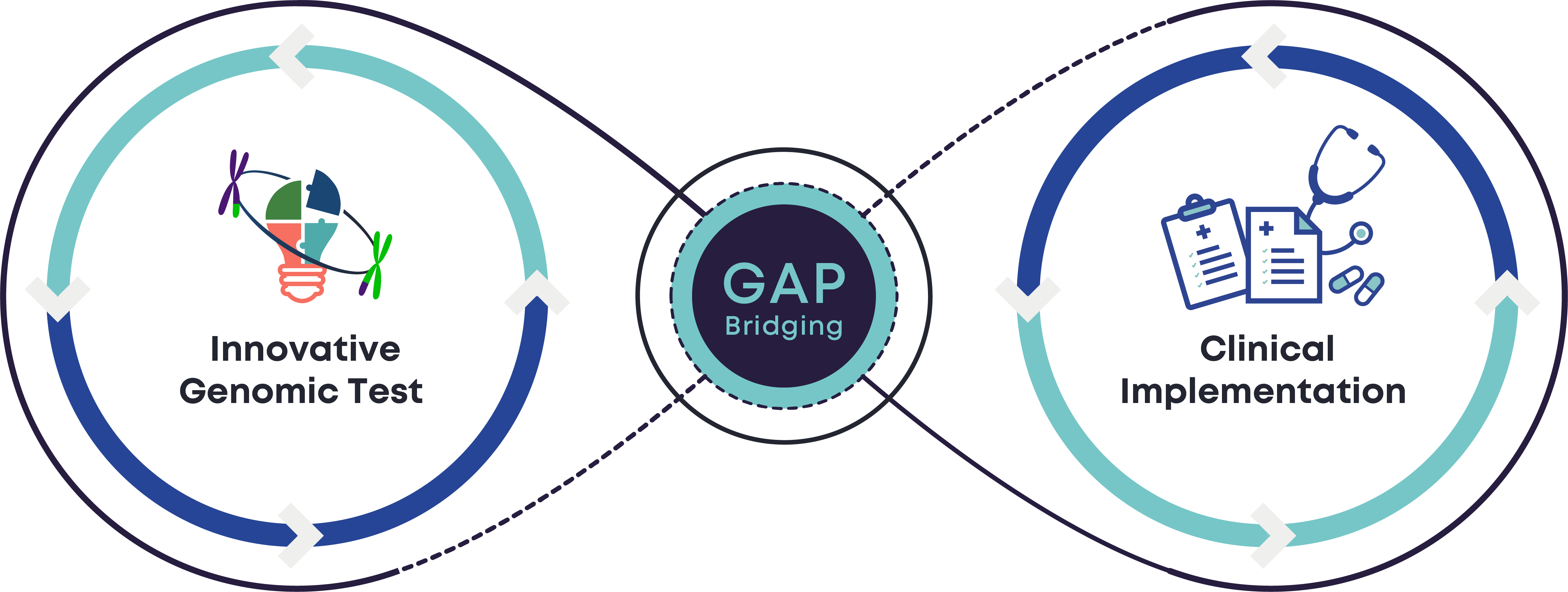
s-Test Genomics mission is to ensure quality while bridging the gap between innovations in the field of genomics and their standardised implementation in clinical services.
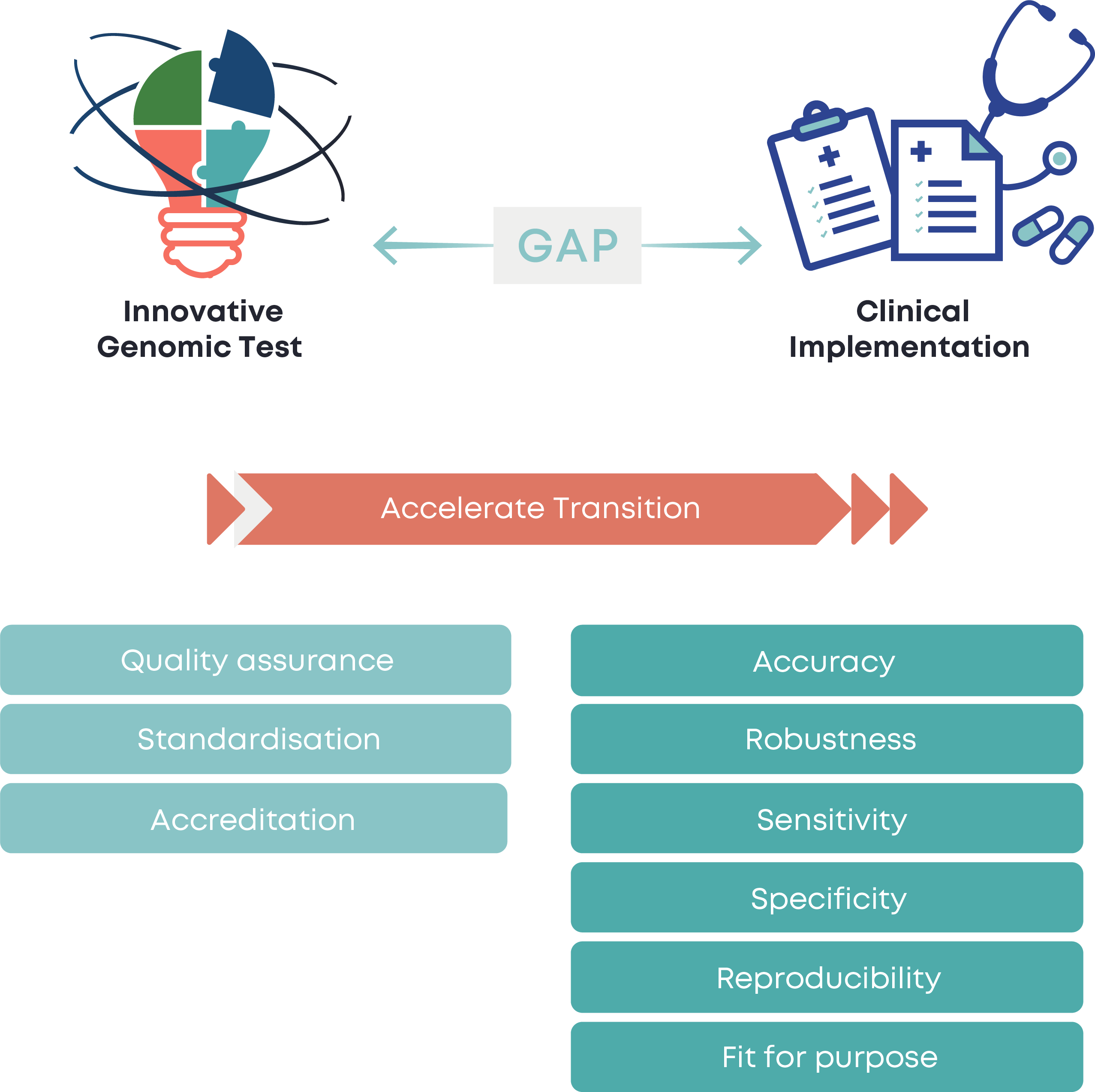
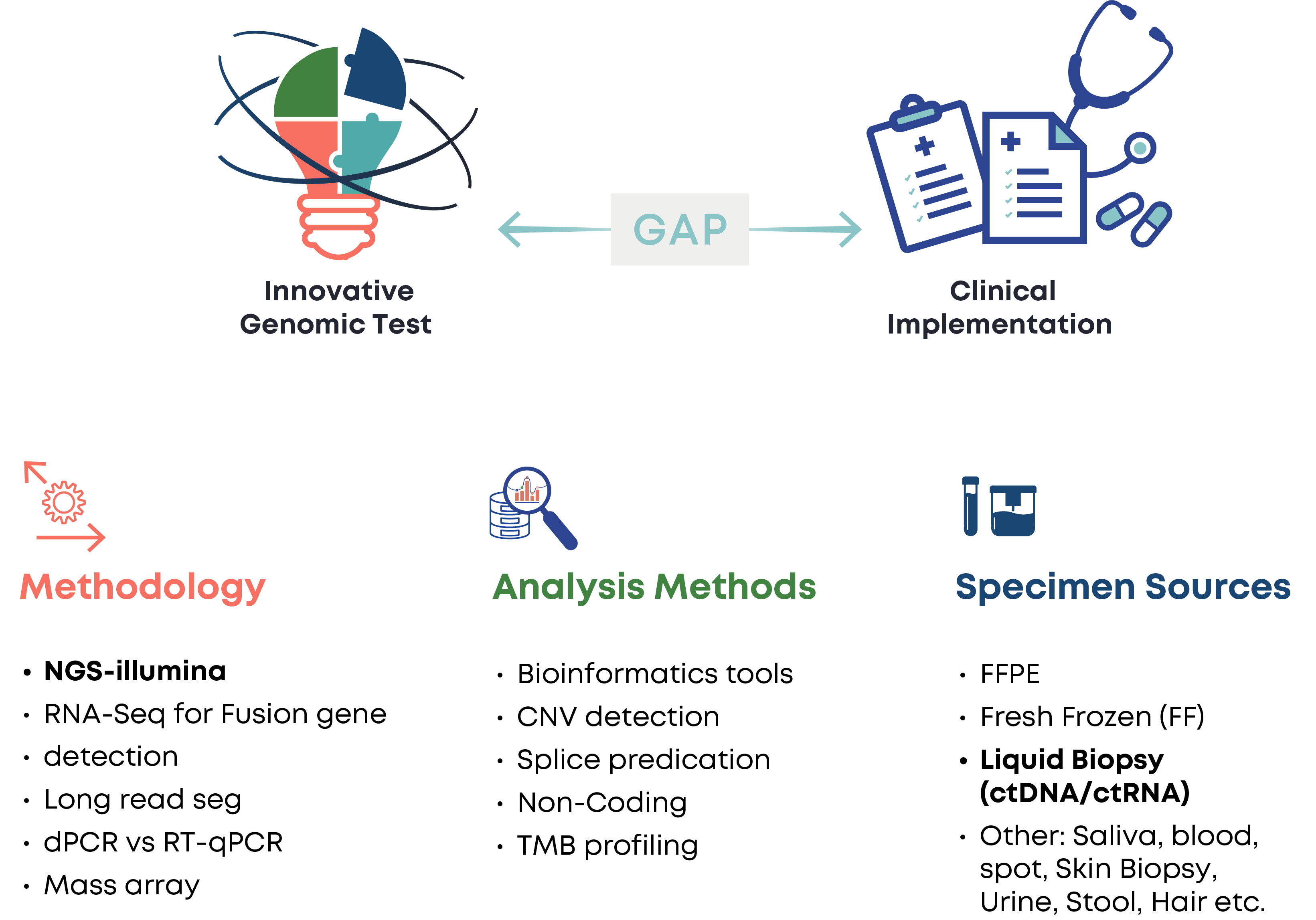
Genomic Testing Standardisation
Accelerated advances in genomic technologies exercise pressure on existing standardisation processes, creating an evident gap between genomic innovations and their clinical utilisation, thus posing a clinical risk.
Coordinated endeavors for new test validation and implementation are needed in the presence of multiple multi-target technologies and solutions for delivering genomic tests.
It is paramount to accelerate bridging the historical lag from innovation to clinical practice through quality implementation and centralised coordination of validation processes.